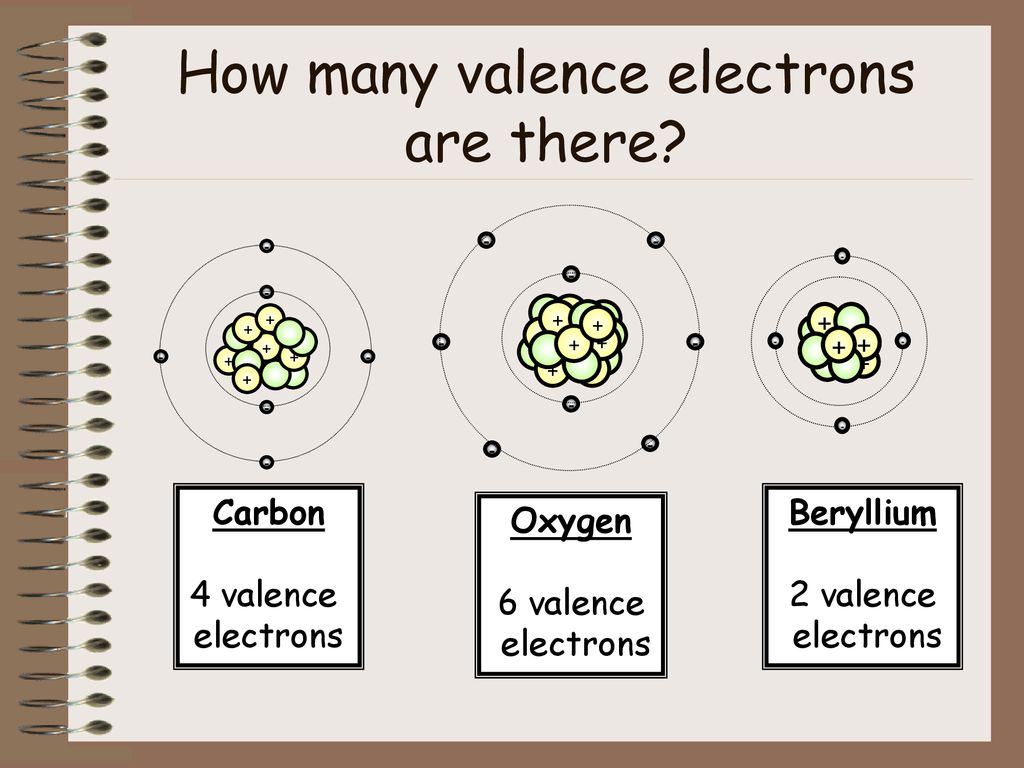
Beryllium has a 2s2 number of valence electrons. Beryllium will lose electrons. It loses two valence electrons. Beryllium is an element that has an atomic number of 4, and is a classified as a metal. How many electrons,number of valence electrons,& number of shell containing electrons does Beryllium Atom have.
Diagrams contain a lot of useful information in a compact format. Diagrams of electrons provide information on the location of specific electrons. We can mark these electrons and indicate what happens to them when an element reacts.
Electron Dot Diagrams


Recall that the valence electrons of an atom are the electrons located in the highest occupied principal energy level. Valence electrons are primarily responsible for the chemical properties of elements. The number of valence electrons can be easily determined from the electron configuration. Several examples from the second period elements are shown in the table below.
| Table (PageIndex{1}) | ||
| Element | Electron Configuration | Number of Valence Electrons |
| lithium | (1s^2 : 2s^1) | 1 |
| beryllium | (1s^2 : 2s^2) | 2 |
| nitrogen | (1s^2 : 2s^2 : 2p^3) | 5 |
| neon | (1s^2 : 2s^2 : 2p^6) | 8 |
In each case, valence electrons are those in the second principal energy level. As one proceeds left to right across a period, the number of valence electrons increases by one. In the (s) block, Group 1 elements have one valence electron, while Group 2 elements have two valence electrons. In the (p) block, the number of valence electrons is equal to the group number minus ten. Group 13 has three valence electrons, Group 14 has four, and so on...up through Group 18 with eight. The eight valence electrons, a full outer (s) and (p) sublevel, give the noble gases their special stability.
When examining chemical bonding, it is necessary to keep track of the valence electrons of each atom. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, has the electron dot diagram below:
[cdot ce{Be} cdot]

Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired. The table below shows the electron dot diagrams for the entire second period.
| Table (PageIndex{2}): Electron Dot Diagrams for the Second Period Elements | |
| Group Number | Electron Dot Diagram |
| 1 | (ce{Li} cdot) |
| 2 | (cdot ce{Be} cdot) |
| 13 | (cdot overset{cdot}{ce{B}} cdot) |
| 14 | (cdot underset{cdot}{overset{cdot}{ce{C}}} cdot) |
| 15 | (cdot underset{cdot}{overset{cdot}{ce{N}}} :) |
| 16 | (: underset{cdot}{overset{cdot}{ce{O}}} :) |
| 17 | (: underset{cdot}{overset{cdot cdot}{ce{F}}} :) |
| 18 | (: underset{cdot cdot}{overset{cdot cdot}{ce{Ne}}} :) |
Beryllium Valence Electron Count
Electron dot diagrams would be the same for each element in the representative element groups. Most transition elements have two valence electrons, though some that have unusual electron configurations have only one.
Summary
The Number Of Valence Electrons In Beryllium
- Electron dot diagrams show the valence electrons for an atom.
- The dot diagrams are the same for each element in the representative element groups.
Contributors and Attributions
The Number Of Valence Electrons In Beryllium
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.

Comments are closed.